How to add a new patient
How to add a new patient
click the "add a new patient / screening failure" button
Please note that it is necessary to save the pre-screening form in order to obtain the patient code. This patient code is unique for the whole study and it is the responsibility of the investigator to know the relationship between this code and the medical record number
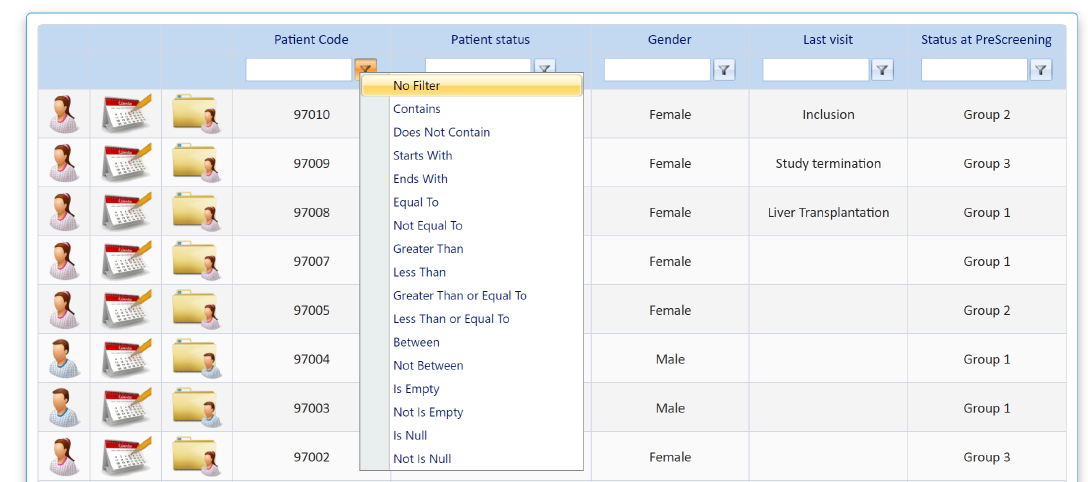
- Once the patient code is available, it will appear in the main screen of patients
- Main screen is ordered by patient code, being the last patient entered, the first of the list
There are basic variables selected to search the patient and filters (funnel-shaped icon next to each variable ) to perform and advanced search
) to perform and advanced search
 Short cuts of the platform
Short cuts of the platform
Please be sure of using the “next“ and “exit without save“ buttons in order to navigate through the platform. Back button of the browser does not ensure that patient’s data are properly saved
 “Calendar icon” is the recommended route during the data entry process. It is a shortcut to the visit schedule
“Calendar icon” is the recommended route during the data entry process. It is a shortcut to the visit schedule
 “Folder icon” is the recommended route during the data cleaning process. It is a shortcut to all the independent forms as well as all the queries
“Folder icon” is the recommended route during the data cleaning process. It is a shortcut to all the independent forms as well as all the queries
 How to add a new patient
How to add a new patient
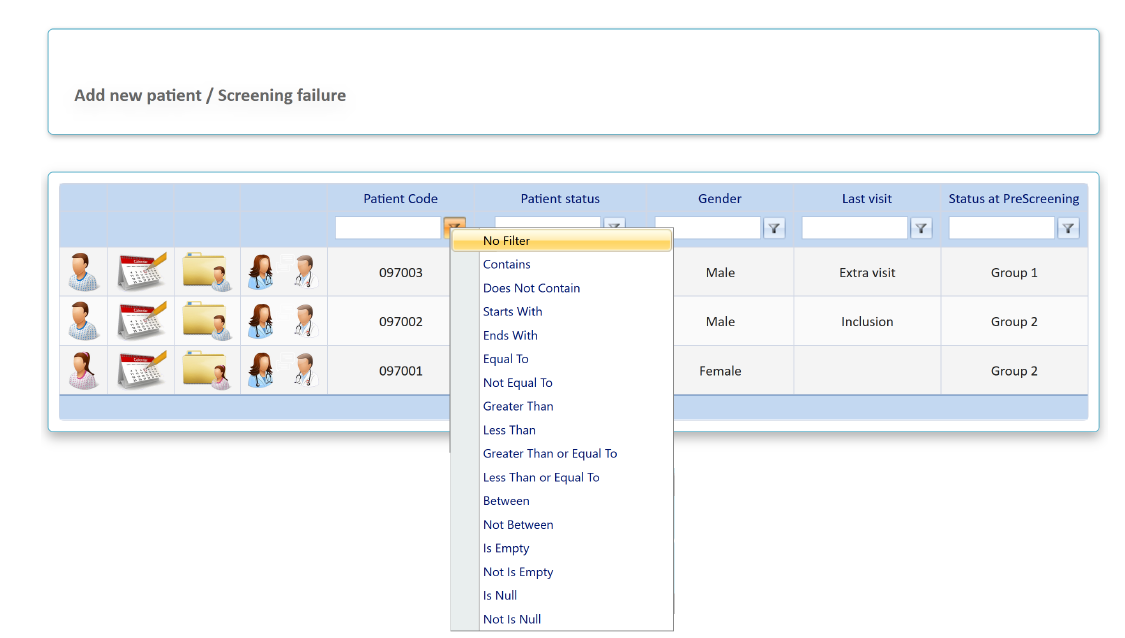
) to perform and advanced search